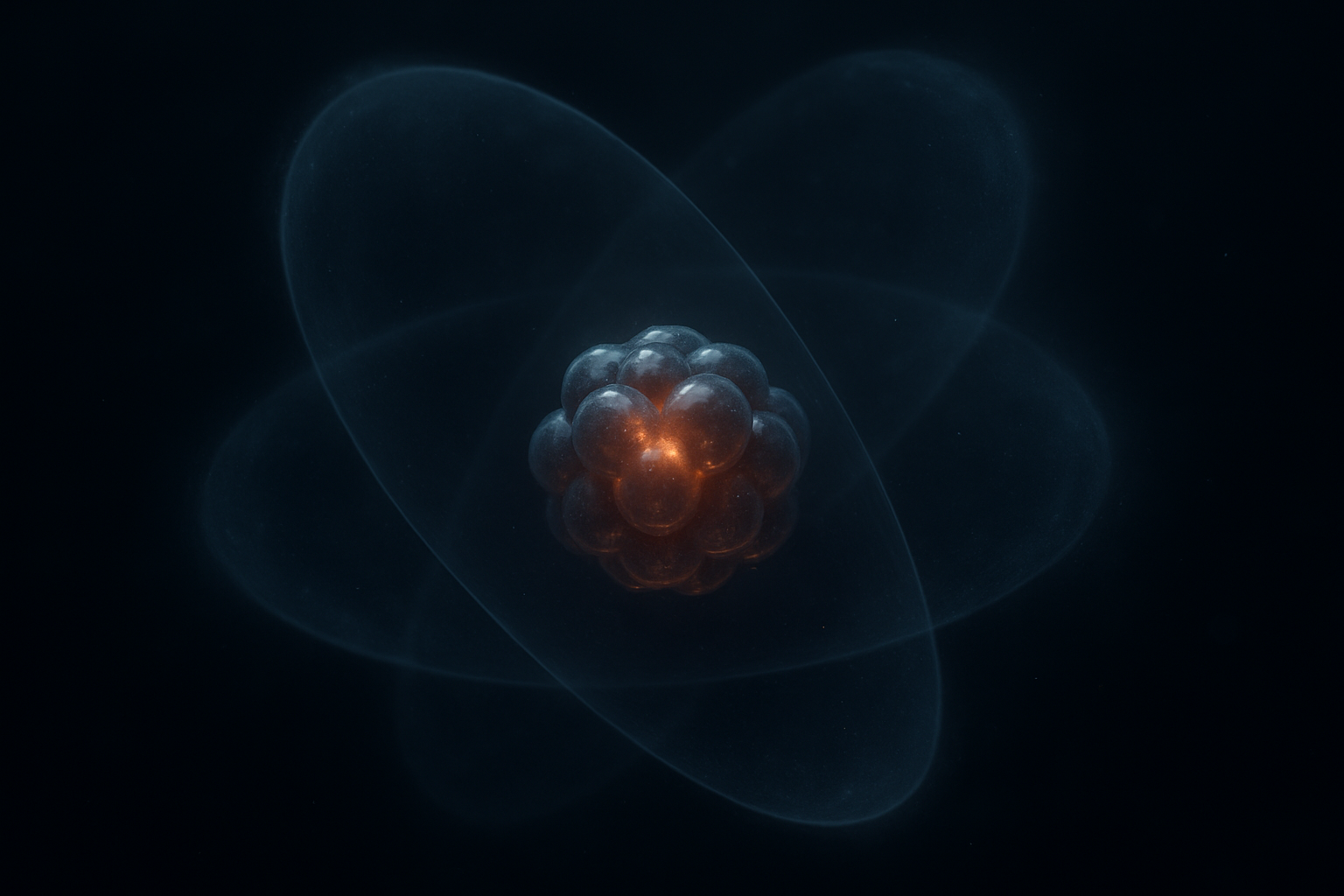
An atom is the smallest building block of all matter. Everything around us — air, water, plants, people, and objects — is made up of atoms. An atom can be imagined as a tiny ball consisting of a nucleus and a shell.
The atomic nucleus contains positively charged protons and uncharged neutrons. Negatively charged electrons move around the nucleus. The number of protons in the nucleus determines which chemical element it is – for example, hydrogen, oxygen, or iron.
Atoms are incredibly small: there are billions of them in a single grain of sand. Nevertheless, they are the basis of everything that exists.
Quantum physics shows that atoms behave very differently from what we are used to in our everyday world. Electrons do not simply move in fixed orbits around the nucleus, but can exist in several states at the same time and only “appear” in one place with a certain probability. In addition, atoms can be entangled with each other in such a way that the behavior of one atom is immediately connected to another – no matter how far apart they are.
These special properties make atoms important building blocks for quantum research. They are the basis for technologies such as quantum computers, quantum communication, and high-precision measuring instruments that go far beyond what classical technology can achieve.